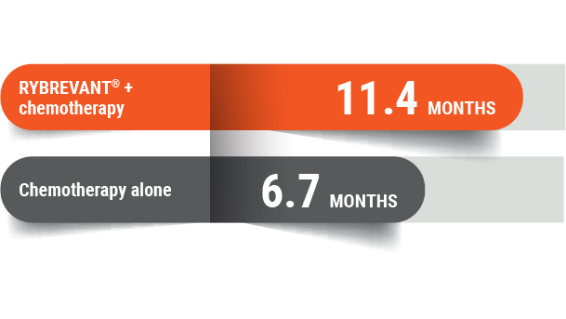
More time without disease worsening
RYBREVANT® + chemotherapy is proven to reduce the risk of cancer growing or spreading by 60% compared with chemotherapy alone
People who received RYBREVANT® + chemotherapy saw almost twice the time without cancer growing or spreading
At 11.4 months, half of the people receiving RYBREVANT® + chemotherapy lived without their cancer growing or spreading, compared with 6.7 months for chemotherapy alone.
About the clinical trial
These results are from a clinical trial where 308 people with advanced NSCLC with received RYBREVANT® in combination with chemotherapy as a first treatment, or received chemotherapy alone as a first treatment. About the people in the clinical trial:
- Most of the people never smoked (58%)
- Over half of the people were women (58%)
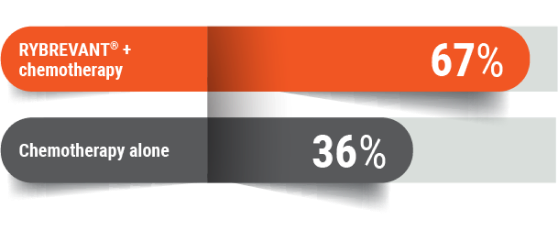
More response to treatment
67% of people responded (tumors got smaller* or disappeared†) with RYBREVANT® + chemotherapy
RYBREVANT® + chemotherapy
Tumors got smaller*: 63%
Tumors disappeared†: 4%
Chemotherapy alone
Tumors got smaller*: 36%
Tumors disappeared†: 1%
*Tumors getting smaller may also be called a partial response. This means the tumor got measurably smaller but is still detectable.
†Tumors disappearing may also be called a complete response. This does not necessarily mean the cancer has been cured.
Chemotherapy = carboplatin + pemetrexed
EGFR+ = mutated epidermal growth factor receptor; NSCLC = non–small cell lung cancer
Side effects
There are side effects that you may experience during treatment with RYBREVANT® + chemotherapy.


Savings & support
Once prescribed a RYBREVANT®-based treatment, connect with a Care Navigator for your support needs.



